This test is run by .
Note that your final mark will not be saved in the system.
Note that your final mark will not be saved in the system.
Chemical bonds, ionic, covalent and metallic MatchUp
Target Level
4-5
Running Total
0
0%
Attempt
1 of 3
Click on a top box, then click on its match below. Or, drag a top box and drop it onto the correct match. Match all pairs before clicking ‘Check’.
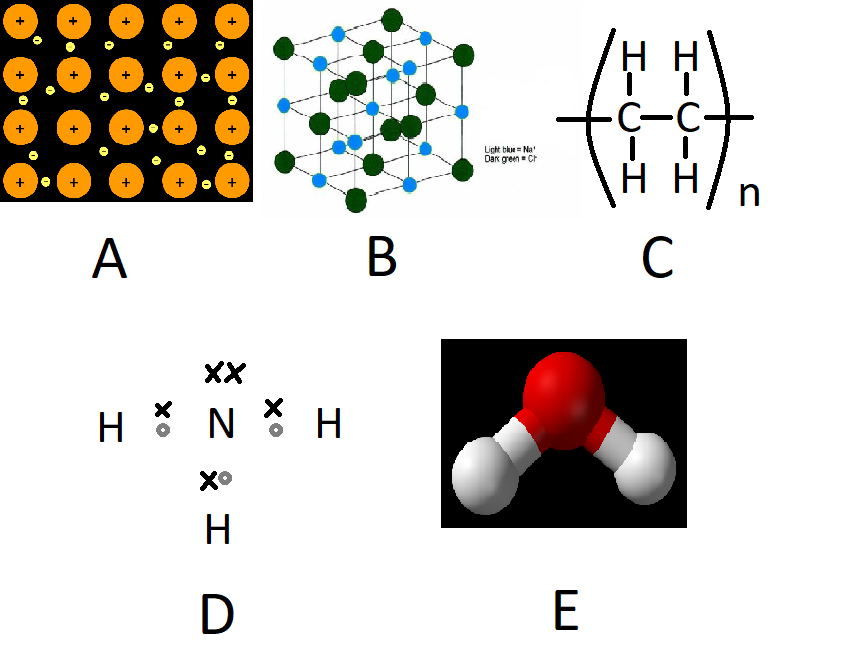
Three
Metals and non-metals together
Ionic
Non-metals
Metallic
B
A
Metals
Covalent
Dot and cross diagram
Ball and stick
Type of representation used to show compound D above
Atom types which show covalent bonding
Atom types which show ionic bonding
Type of representation used to show compound E above
Atom types which lose electrons in metallic bonding
Type of bonding where atoms share pairs of electrons
Type of bonding involving oppositely charged ions
Compound shown above which has delocalised electrons
Compound shown above which is ionic
Number of sodium ions per three chloride ions in substance B above
Type of bonding where atoms share delocalised electrons