This test is run by .
Note that your final mark will not be saved in the system.
Note that your final mark will not be saved in the system.
5.1.1/5.1.2 How fast and how far? MatchUp
Target Level
C
Running Total
0
0%
Attempt
1 of 3
Click on a top box, then click on its match below. Or, drag a top box and drop it onto the correct match. Match all pairs before clicking ‘Check’.
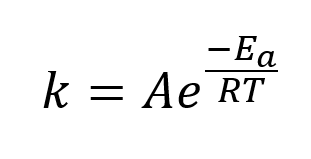
Pa
order of reaction with respect to reactant
equilibrium constant
mol dm−3
rate constant
Arrenhius equation
mol dm−3 s−1
Le Chatelier's principle
rate equation
unit of concentration
unit of rate of reaction
mathematical relationship between rate of reaction, k and concentration of reactants
quantity represented by k in the equation above
m or n in rate = k[A]m[B]n
quantity represented by K (as in Kc or Kp)
mathematical relationship between k, activation energy and temperature (shown above)
concept predicting how chemical equilibria respond to changes in conditions
common unit of pressure