This test is run by .
Note that your final mark will not be saved in the system.
Note that your final mark will not be saved in the system.
Chemical equilibria and redox equations MatchUp
Target Level
C
Running Total
0
0%
Attempt
1 of 3
Click on a top box, then click on its match below. Or, drag a top box and drop it onto the correct match. Match all pairs before clicking ‘Check’.
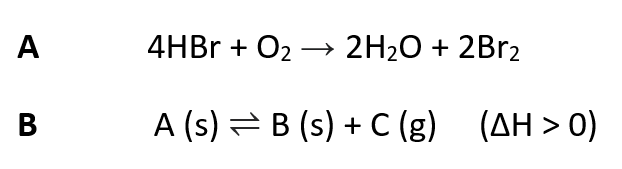
increases
reduction
oxidation
stays the same
chemical equilibrium
homogeneous reaction
decreases
redox reaction
heterogeneous reaction
process involving loss of electrons
process involving gain of electrons
reversible reaction where rates of forward and reverse reactions are the same, and where the concentrations of all reactants and products remain the same
reaction where at least one species gains electrons and at least one species loses electrons
reaction where the reactants and products are all in the same phase
reaction where the reactants and products are not all in the same phase
in reaction A (above), the oxidation state of hydrogen...
in reaction A (above), the oxidation state of oxygen...
in reaction A (above), the oxidation state of bromine...