This test is run by .
Note that your final mark will not be saved in the system.
Note that your final mark will not be saved in the system.
Transition metals MatchUp
Target Level
C
Running Total
0
0%
Attempt
1 of 3
Click on a top box, then click on its match below. Or, drag a top box and drop it onto the correct match. Match all pairs before clicking ‘Check’.
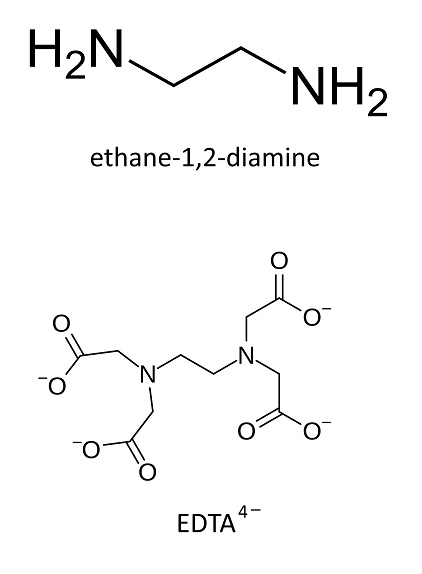
bidentate ligand
4s subshells
multidentate ligand
tetrahedral
heterogeneous
cis−trans
optical
homogeneous
3d subshells
octahedral
monodentate ligand
water is a...
ethane-1,2-diamine (shown above) is a...
EDTA4− (shown above) is a...
3d transition metals have, or form ions with, incomplete...
subshells that electrons are first lost from when 3d transition metals are ionised
common geometry for complexes with larger, charged ligands
common geometry for complexes with smaller, neutral ligands
type of isomerism sometimes displayed by square planar complexes and by octahedral complexes with monodentate ligands
type of isomerism sometimes displayed by tetrahedral complexes and by octahedral complexes with bidentate ligands
catalyst in a different phase to the reactants
catalyst in the same phase as the reactants